Hypertrophic Obstructive Cardiomyopathy (HOCM)
Pathophysiology:
- Genetically determined primary cardiac muscle disease.
- 60-70% caused by mutations in sarcomere genes.
- Characterized by LV Hypertrophy in the absence of other aetiologies.
- Asymmetric LV hypertrophy especially the interventricular septum is the Hallmark of HOCM
- There is diastolic dysfunction and varying degrees of dynamic LVOT obstruction.
- Myocardial ischaemia may also be present.
- Most commonly results from hypertrophy of the basal septum with mitral-septal contact secondary to SAM of the AML causing (posteriorly directed) MR caused by incomplete leaflet coaptation.
- Venturi or Drag Effect:
- High LVOT blood velocities which pull the AML towards the septum (SAM)
- Causing mechanical impedance to blood flow.
- Create a pressure gradient between the LV and the aorta at mid-systole.
- High LVOT blood velocities which pull the AML towards the septum (SAM)
- Associated with a variety of anomalies of the mitral valve apparatus:
- Elongated AML and PML
- Anterolateral papillary muscle displacement.
- Anterolateral papillary muscle insertion into the middle of AML
- Anomalous chords insertion into the middle of the AML.
- Subsets of HOCM:
- Basal Septal Hypertrophy:
- Associated SAM
- LVOT Obstruction
- Pure Mid-ventricular Obstruction:
- Septal contact with papillary muscles causes obstruction to blood flow.
- MV leaflets do not contribute to the obstruction
- There is no SAM
- Causes high intra-ventricular pressure in the apical area and in combination with subendocardial ischaemia, it predisposes to apical aneurysm.
- Septal Myectomy is performed through an apical incision.
- this can be combined with subaortic approach to adequately relieve obstruction.
- Apical non-obstructive HOCM:
- Non-obstructive and most are asymptomatic.
- Some may suffer from severe diastolic dysfunction.
- Severe LV hypertrophy with very small ventricular cavity reducing LV diastolic filling.
- Apical Myectomy aiming to enlarge the LV volume:
- Improves haemodynamics
- Improves functional capacity
- May delay or eliminate the need for Heart Transplant
- Basal Septal Hypertrophy:
Clinical Presentation:
- Asymptomatic:
- Non-obstructive HOCM.
- Good Prognosis
- 10% progress to NYHA class III/IV symptoms.
- Survival is comparable with age matched controls.
- Symptomatic Heart Failure caused by diastolic dysfunction, MR and LVOT obstruction.
- 70% have resting or provoked LVOT obstruction.
- 25% have resting LVOT gradient >30mmHg.
- Maximum subaortic pressure gradient >30mmHg
- Resting or after provocation.
- Diagnostic
- Predicts HF and poor prognosis
- Syncope due to reduced CO from LVOT obstruction.
- Angina:
- Coronary microvascular abnormalities.
- Inadequate capillary densities for the degree of hypertrophy.
- Arrhythmias:
- Supraventricular and ventricular arrhythmias.
- Sudden cardiac death 0.5 - 1.5% / year
- more common in patient >30 years old.
- Rare > 60 years of age.
- Increased risk when associated with:
- History of Cardiac arrest.
- Family history of HOCM-related sudden death.
- Sustained VT
- Recurrent prolonged episodes of non-sustained VT.
- Massive LVH >30mm
- Apical LV aneurysm.
- LGE >15% of LV mass.
- End-stage HOCM with EF <30% and an Apical LV aneurysm.
- Atrial Fibrillation:
- in 20% due to left atrial dilatation.
- Common in advanced HF and systolic dysfunction.
- Poorly tolerated due to loss of atrial kick with severe LVH causing reduced CO.
- A predictor of poor outcome.
- Treated with:
- amiodarone.
- anticoagulation.
- Catheter AF ablation.
- Maze procedure at the time of surgical myectomy.
- Advanced HF with systolic dysfunction that may require Heart Transplant.
Preoperative Evaluation:
- TTE:
- Most commonly used imaging to assess:
- LV morphology including the subaortic area.
- Haemodynamics by Doppler.
- identify SAM and associated posteriorly directed MR jet.
- Intrinsic mitral valve disease or anomalies of the mitral apparatus.
- Intra-operative TOE to further assess the Mitral Valve.
- Most commonly used imaging to assess:
- Provocative Tests:
- For latent LVOT obstruction in patients with exertional symptoms patients with no or minimal LVOT gradients at rest.
- Valsalva manoeuvres, amyl nitrate inhalation or simple exercise to elicit outflow tract obstruction murmur.
- Confirmed using these manoeuvres with TTE.
- Failure to confirm LVOT obstruction will necessitate further tests.
- Cardiac Catheterisation:
- Suspected labile LVOT obstruction not confirmed on TTE.
- Provocative Tests:
- Isoproterenol stimulation.
- Nitrates infusion.
- Eliciting PVC.
- Documentation of LVOT gradient >50mmHg confirms the diagnosis.
Management:
- Consensus guidelines recommend initial medical therapy for Obstructive HOCM with:
- Beta Blockers.
- Calcium channel blockers
- +/- Disopyramide (Class IA anti arrhythmic Na Channel Blocker)
- Invasive Relief of LVOT obstruction:
- those who continue to be symptomatic under optimal therapy:
- with impaired functional capacity.
- Subaortic pressure gradient >50mmHg (at rest or after provocation)
- Do not tolerate side effects of medications.
- those who continue to be symptomatic under optimal therapy:
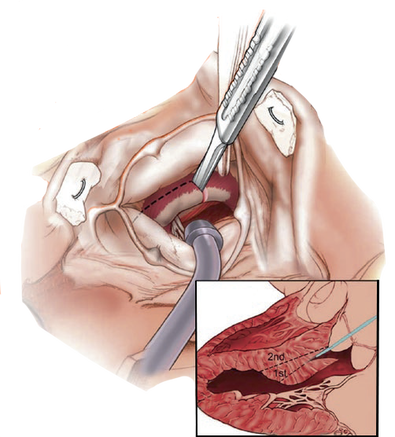
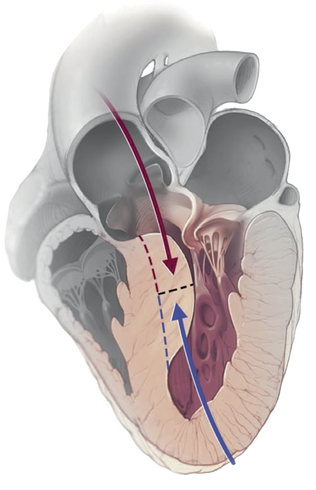
- Transaortic septal myectomy is the standard treatment for septal reduction.
- Survival after myectomy for obstructive HOCM is similar to non-obstructive HOCM
- Survival after myectomy is superior to unoperated obstructive HOCM.
- Evidence for beneficial effect on late survival:
- Reduced incidence of ICD discharges.
- Diminished MR
- Improved PHT
- Degree of reversed myocardial remodelling.
- It is also possible to perform for other subsets of HOCM.
- Apical Myectomy:
- Used for pure midventricular obstruction
- To enlarge LV volume and improve LV Filling.
- Provides immediate relief of symptoms.
- Has excellent long-term outcome.
- Eliminate or delays the need for Transplant
- Can be combined with Subaortic Myectomy to achieve adequate relief of obstruction.
- Used for pure midventricular obstruction
- Contraindications to Surgical Myectomy:
- General contraindications for Cardiac Surgery:
- Advanced Age.
- Frailty.
- Multiple Comorbidities that limit expected survival.
- Alcohol (Ethanol) Septal Ablation: may be an alternative choice for High Risk Patients with contraindications to surgical myectomy.
- General contraindications for Cardiac Surgery:
Alcohol (Ethanol) Septal Ablation:
- Also known as:
- Percutaneous Transluminal Septal Myocardial Ablation
- Trans-coronary ablation of Septal Hypertrophy.
- Non-Surgical Septal Reduction Therapy.
- Relieves obstruction by creating a localized myocardial infarction in the basal septal muscle.
- Following remodelling of this area, the LVOT is widened, relieving the obstruction.
- Improves symptoms and increases exercise capacity.
- Benefits are comparable in younger and older (age >60 year) patients
- Procedure:
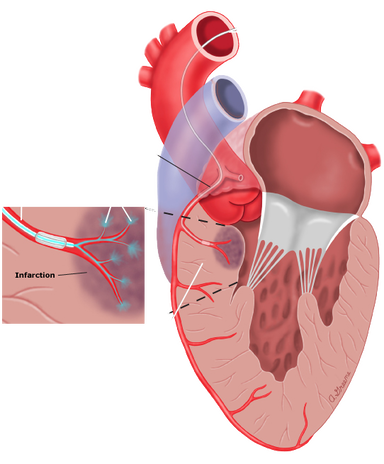
- During Cardiac Catherization the First and Second septal perforator branches of the LAD are injected with Ethanol to create a localized controlled MI in the basal septum
- Remodelling over time will widen the LVOT and relieves the obstruction.
- A Large scar is created (10%) of total LV mass.
- MCE (Myocardial Contrast Echocardiography):
- Delineate the size of the septal vascular area
- Predict the infarct size.
- May lower the risk of CHB requiring PPM.
- Drawbacks:
- Repeat Ablation may be required in 6%.
- Conflicting data regarding efficacy in marked septal thickness:
- Survival is significantly worse in septal thickness >25mm
- Better long term survival in septal thickness <16mm but with increase early complication.
- Complications:
- Coronary artery dissection (LAD 1.8%).
- Pericardial effusion (0.6%)
- Large MI secondary to escape of ethanol from the target vessel to another coronary artery (usually the LAD).
- Complete Heart Block ( requiring PPM 10%) (More RBBB being supplied by the septal perforators)
- Ventricular Tachyarrhythmia
- Arrhythmic Death (VF 2.2%) (there is no definitive evidence that septal ablation increased sudden cardiac death)
- VSD ( should not be performed with septal thickness <15mm)
Depiction of alcohol (ethanol) septal ablation
© 2021 UpToDate, Inc. and/or its affiliates. (Graphic 78141 Version 6.0)
© 2021 UpToDate, Inc. and/or its affiliates. (Graphic 78141 Version 6.0)
Comparison between Surgical Myectomy and Alcohol Ablation:
- Uncertain effectiveness in massive LV hypertrophy (>30mm), surgical myectomy should be considered instead.
- Surgical Myectomy results in significantly lower residual LVOT gradients.
- No significant difference in long-term survival.
Surgical Myectomy |
Alcohol Septal Ablation |
Higher success rate 90-95% |
Success Rate 80-90% |
Immediate sustained relief of LVOT obstruction and concomitant MR |
Delay up to 3 months in improvement |
Ability to obtain tissue biopsy |
No Biopsy |
Lower incidence of CHB 3% |
Incidence of CHB 10% |
Better Symptom resolution |
|
Proven long-term efficacy (>20 years) |
|
More chances of success in massive septal hypertrophy (>30mm) |
Avoided in massive septal hypertrophy (>30mm) |
No damage distal to target area |
Risk of myocardial damage distal to the target area |
Ability to perform concomitant procedures (AF ablation, CABG, MR repair/replacement) |
Avoidance of Sternotomy and CPB especially in high risk patient, shorter hospital stay, lower costs. |
May reduce the risk of Sudden Cardiac Death and ICD discharges |
Lower Risk of VSD |
HOCM: Hypertrophic Obstructive Cardiomyopathy, LV: Left Ventricle, LVOT: Left Ventricular Outflow Tract, CO: Cardiac Output
AML: Anterior Mitral Leaflet, PML: Posterior Mitral Leaflet, MR: Mitral Regurgitation, LGE: Late Gadolinium Enhancement, PHT: Pulmonary hypertension, CHB: Complete Heart Block, PPM: Permanent Pacemaker
AML: Anterior Mitral Leaflet, PML: Posterior Mitral Leaflet, MR: Mitral Regurgitation, LGE: Late Gadolinium Enhancement, PHT: Pulmonary hypertension, CHB: Complete Heart Block, PPM: Permanent Pacemaker